Paul Turner, Ph.D.: When antibiotics came about, it really was seen as a set of wonder drugs, and they were. Suddenly, we had this drug arsenal that we could use to prevent mortality in humans worldwide. So the problem was that we didn’t anticipate that if you expose bacteria to antibiotics in a very widespread way, you’re going to exert what’s called selection pressure.
Bacteria can evolve very, very quickly. Under the right conditions, they undergo multiple generations in a single day. So bacteria are growing and if they mutate they can evolve to be better resistant to antibiotics that they’re encountering in the environment. And this is what has led to the current antibiotic resistance crisis.
Pseudomonas aeruginosa is a very mobile bacterium, and it’s exceedingly common on earth. Maybe you go swimming in a lake or even the ocean, and then suddenly you get a bacterial infection. It has many worrisome features. So it has all these little structures around the surface that look like tiny hairs, and these are called pili. And then they also have something called flagella. They’re almost like a whipping tail that helps the bacteria move quickly through liquids.
Worse yet, they have very efficient ways of removing antibiotics from the cell if they get in. These are called efflux pumps. Antibiotics that make it inside of that cell, these efflux pumps allow the bacteria to actively pump the antibiotics out. Over the course of a chronic infection, with a bacterium like Pseudomonas aeruginosa, this could lead to organ failure, and this can unfortunately lead to patient mortality.
We’re running out of options for antibiotics to use in the clinic. I really got interested in the old idea of phage therapy. So a phage is literally a virus of bacteria. Phages have been known for a long period of time. In roughly 1917, Felix Durrell, who was the discoverer of phages, he saw that there was something that was capable of killing bacteria very efficiently.
So phages come in many flavors and some of the most interesting ones look almost like a lunar lander. These phages, they’re kind of like predators. They’ll come into a bacterial cell and make it into basically a production factory for baby phages, and then they’ll explode the cell, and they’ll go and infect other bacterial cells. And as I studied that, I thought, well, whoa, so it’s literally possible to use phages to kill the bacteria that we’re particularly worried about.
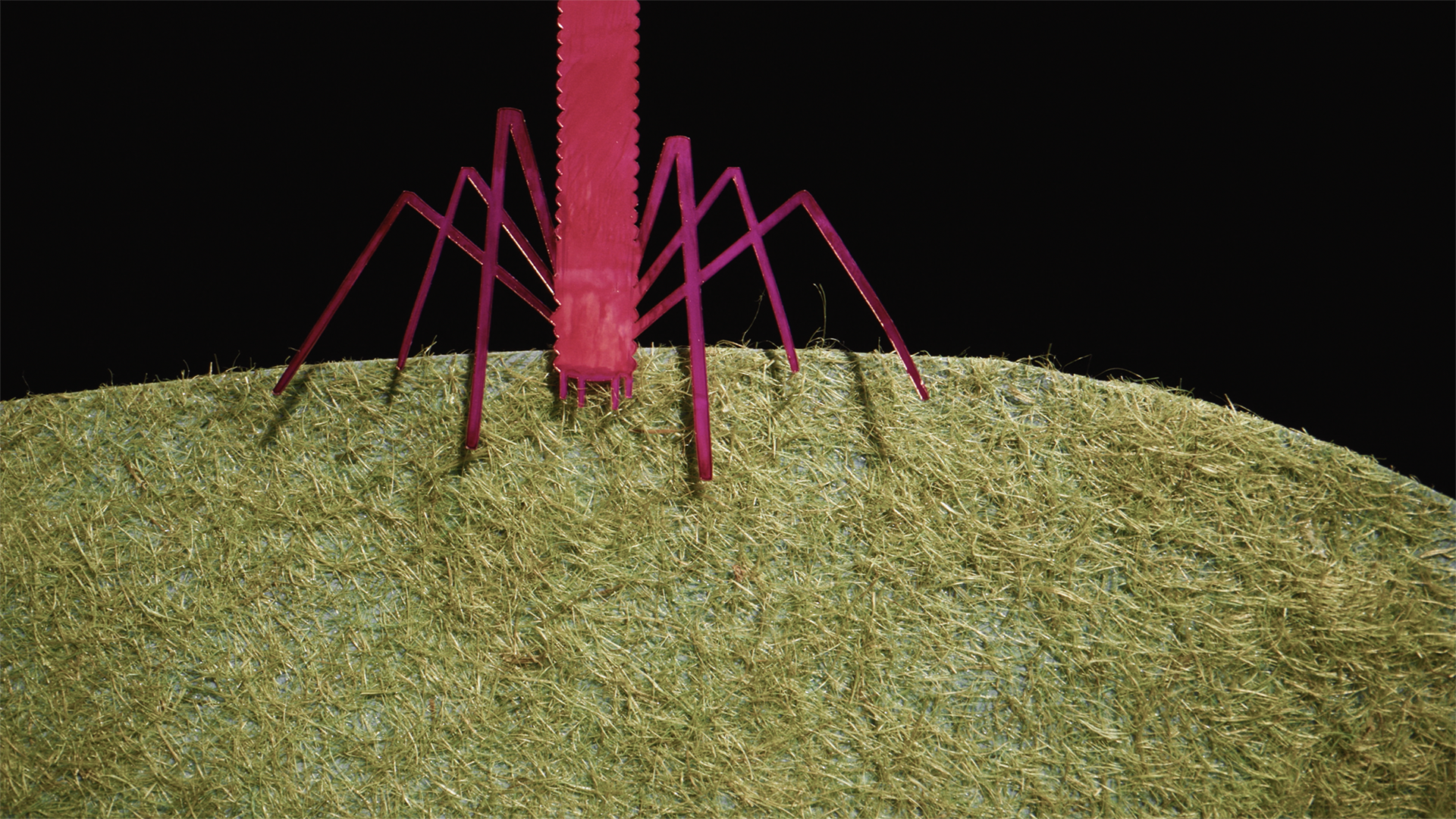
So modern day phage therapy really rests upon one thing, and that is phage discovery. That means that we often go out into the natural world, to try and find the right ones. So one of our favorite phages that we discovered early on when we did phage therapy, we abbreviated it as OMKO1, and that’s stood for outer membrane knockout one, it was the first phage that we discovered that was specific to Pseudomonas aeruginosa. So what we did was deploy this phage, that’s going to attach to these efflux pumps, get into the bacterial cells and kill them, because that’s what phages do.
In order to overcome that problem, we were predicting that the bacteria should evolve, and they should evolve in a particular, predictable way. They should change so that that protein that the phages are binding to is no longer present. And if that happened, they would remove the efflux pumps in order to solve the phage problem.
So they may gain phage resistance, and therefore withstand the therapy that we were deploying, but they would be suddenly vulnerable to antibiotics again because those antibiotics could not be pushed out of the cell any longer. That’s the double-edged sword. We kill it in the classic sense, but we don’t worry when the bacteria evolve resistance to phage attack, because it makes them automatically vulnerable to antibiotics.

What does that give us in the end? Antibiotics are wonderful, but they are chemical entities. They don’t change through evolution, and instead, phages have the power to do so. So this is what we’re hoping to really tap into. Phages co-evolve and remain potent as killers of bacteria. And in this way, we’re not only using a new approach, we’re actually keeping existing drugs useful again in the clinic and in the hospital.
Chan, B., Sistrom, M., Wertz, J. et al. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep 6, 26717 (2016). doi: 10.1038/srep26717
Kortright KE, Chan BK, Koff JL, Turner PE. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe. 2019 Feb 13;25(2):219-232. doi: 10.1016/j.chom.2019.01.014.
For the film:
Directors: Ruth Lichtman & Sharon Shattuck
Producers: Sharon Shattuck, Ruth Lichtman, Regina Sobel
Cinematographers: Taylor Dekker (Turner lab), Adam Wissing (puppetry)
Editor: Ruth Lichtman
Puppeteers: Shayna Strype, Emma Wiseman, Al Mullen, Ruth Lichtman, Sharon Shattuck
Associate Producer: Lee Rossoff
Original Music: Jordan John Parker
Colorist: Zachary Halberd
Sound design & mix: Richard Hamilton, Dragonfly Audio Post
Graphics & Animation: Sharon Shattuck
For the Science Communication Lab:
Senior Producers: Shannon Behrman and Regina Sobel
Executive Producers: Sarah Goodwin and Elliot Kirschner